 The growth of research and innovations in the area of small molecules is stagnant now, so researchers have revived their interest in the development of specified biologics. These specified biologics include therapeutic DNA-derived products, monoclonal antibodies for in-vivo use, synthetic peptides and therapeutic DNA plasmid products. Amongst these, synthetic peptides are poised between traditional small molecules and therapeutic proteins.
The growth of research and innovations in the area of small molecules is stagnant now, so researchers have revived their interest in the development of specified biologics. These specified biologics include therapeutic DNA-derived products, monoclonal antibodies for in-vivo use, synthetic peptides and therapeutic DNA plasmid products. Amongst these, synthetic peptides are poised between traditional small molecules and therapeutic proteins.
Regulatory agencies are defining synthesised polypeptides as a chain of greater than 40 amino acids but less than 100 amino acids in size (synthetic proteins) and synthetic peptides as a chain of 40 amino acids or less linked by amide bond and synthesized chemically. Synthetic peptides are undergoing renaissance as despite of being biologics they are regulated as a traditional small molecule.
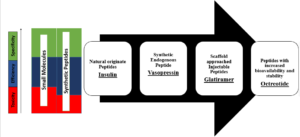
Initially, Peptides like Insulin and calcitonin were isolated from natural origin and proved to be the life saving medicines. But the development of peptides as drugs has been limited due to their shorter half-life, rapid degradation and high levels of clearance. Moreover, low gastrointestinal stability and poor permeability reduces their oral bioavailability significantly.
After the success story of natural peptides and with the advancement in characterization of proteins and peptides, synthetic peptides like vasopressin and oxytocin were evolved with a new synthetic strategy. The novel approaches like stapling, cyclization, N-methylation and glycosylation have resulted in a large number of peptide-based drugs which are more stable to pH and temperature changes, having increased resistance to proteolytic degradation and reduced renal clearance being marketed now.
As compared to therapeutic protein based biologics, Synthetic peptides can be produced in large quantities at low cost as a pure and defined entity which eliminates variability in biological results and their use is devoid of infection and immunogenic risks. Synthetic peptides can also be diversely formulated as injectable (Teriperatide IV injection – Forteo; Octerotide acetate – Bunfezia Pen), sustained release depot (Octreotide acetate – Sandostatin LAR) and oral tablets (Semaglutide – Rybelsus or capsules (Octreotide acetate – Mycapssa).
In contrast to small molecules, peptides are therapeutically more specific and effective in the treatment of critical illness. They are incresingly indicated for the medical management of cancer (Octreotide, Carfilzomib, Ixazomib), Diabetes mellitus (Liraglutide, Semaglutide), Osteoporosis (Teriperatide, Abaloperatide), cardiovascular disease (Eptifibatide), hormonal dysfunction (Cetrorelix, Ganirelix) and infectitious disease (Bulevirtide).
Needless to say, synthetic peptides are emerging as a potential and preferable drug candidate in the field of medical research as they are chemically synthesized in bulk amount and easily modified to introduce anchoring units to tune their binding to the surfaces without significant loss of biological activity.
Author: Ms Shikha Patel, Assistant Professor, Department of Pharmaceutical Analysis